Ask Science
Ask a science question, get a science answer.
Community Rules
Rule 1: Be respectful and inclusive.
Treat others with respect, and maintain a positive atmosphere.
Rule 2: No harassment, hate speech, bigotry, or trolling.
Avoid any form of harassment, hate speech, bigotry, or offensive behavior.
Rule 3: Engage in constructive discussions.
Contribute to meaningful and constructive discussions that enhance scientific understanding.
Rule 4: No AI-generated answers.
Strictly prohibit the use of AI-generated answers. Providing answers generated by AI systems is not allowed and may result in a ban.
Rule 5: Follow guidelines and moderators' instructions.
Adhere to community guidelines and comply with instructions given by moderators.
Rule 6: Use appropriate language and tone.
Communicate using suitable language and maintain a professional and respectful tone.
Rule 7: Report violations.
Report any violations of the community rules to the moderators for appropriate action.
Rule 8: Foster a continuous learning environment.
Encourage a continuous learning environment where members can share knowledge and engage in scientific discussions.
Rule 9: Source required for answers.
Provide credible sources for answers. Failure to include a source may result in the removal of the answer to ensure information reliability.
By adhering to these rules, we create a welcoming and informative environment where science-related questions receive accurate and credible answers. Thank you for your cooperation in making the Ask Science community a valuable resource for scientific knowledge.
We retain the discretion to modify the rules as we deem necessary.
view the rest of the comments
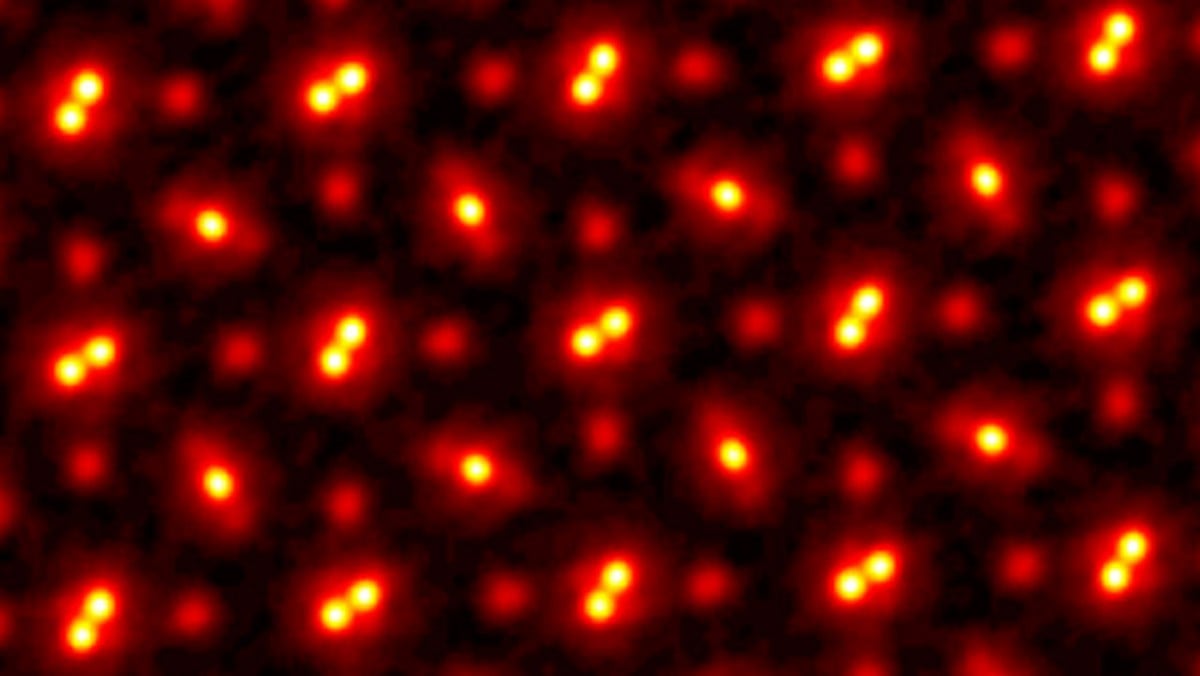
The brighter spots are the nuclei of the Pr, Sc, and O atoms, which are reflecting the electrons of the scanning beams (because they're comparatively much heavier).
The space in between the nuclei is where the electrons from all of the atoms are. Because the atoms are bound as PrScO~3~, the electrons are shared and not really part of any one particular atom or other.
Technically all of it is "the atoms" because the electrons are part of the structure as much as the protons and neutrons.
This diagram in the article is helpful:
The drawing in the lower right shows how the atoms are arranged. The double spots are the nuclei of two Pr atoms very close together. The slightly fainter, elongated spots are actually ScO~2~ that is arranged as O-Sc-O. The fainter single spots are the other O nuclei that fill out the PrScO~3~ structure.
That is only sort of true - this image is not made of electrons reflected by the nuclei. These are results from TEM imaging, so Transmission Electron Microscopy. The electron detector is placed behind the sample.
What you are describing is SEM - Scanning Electron Microscopy - in that case, the detector can be placed above the sample, for example (but not limited to) circularly around the beam to measure the backscattered electrons
In TEM the samples are cut into very thin slices (in the picture you posted it is said to be between 0.8nm - 30nm) and the crystal lattice acts as a diffraction grating for the electron beam. The diffraction pattern can be then used to reconstruct the crystal lattice structure.
So the spots are the nuclei and not the electron cloud? Wow! This is waaaaay smaller imaging than i was thinking it was!
This picture shows the influence of the nuclei, not the nuclei themselves. The nuclei are much smaller. If you throw an electron at an atom, the nucleus will change that electron's direction even if it doesn't hit it, just by being close.
The yellow areas are the 'shades' of the nuclei, but do not reflect their actual size. The lattice constant of the crystal according to the figure is 59 pm = 59 e-12 m, which is the horizontal or vertical distance you see between two of the Pr couples. The actual size of a nucleus would be of order ~ 10 fm = ~ 10 e-15 m.
So, this image was made with a scanning electron microscope - actually several arranged in a grid somewhat similar to a digital camera sensor. Basically the way this works is that a beam of electrons (kind of like a laser, but electrons instead of photons) is fired at the material being scanned. The electrons bounce off of anything heavier than they are, such as the protons and neutrons in the nucleus (electrons are about 1/2000 of the mass of a proton). Some of the electrons bounce back into the detection grid of the microscope.
So the bright spots are where the electrons bounced off of the nuclei back into the detection grid. You can't really get an image of an electron cloud with an electron microscope because electrons are all the same mass, so if you hit one with another one they both move away in random directions (hitting one billiard ball with another). Comparatively hitting a proton with an electron isn't strong enough to move the proton very much (hitting a house with a billiard ball).
I should also say that this is a simplification because protons, neutrons and electrons don't really exist as physical ball-shaped particles, but as probability waves. Arvin Ash gives the best explanation of this that I've seen.
The upshot of all that is that the bright spots in the image show where the protons and neutrons of the atoms were most likely to be during the scanning (it's really difficult to talk about anything absolute at this scale, everything is probabilistic).
Also yes, this image is a very tiny area, literally a few atoms across. It's very impressive, and it basically amounts to visual proof that what we believe to be true about molecular bonding is true because the picture actually shows what the theory predicts.
Bro must've written the article
Nah, I just read a lot of quantum mechanics stuff because the world we live in is complex and sort of illusory from this point of view and I think it's fascinating. I do recommend the Wikipedia article on the standard model of particle physics and this video by AlphaPhoenix about using a scanning transmission electron microscope.
Looking at that diagram, it would be really cool if they could use a second beam to generate a 3D image that you could explore in VR.